Both quartz and diamond are beautiful gemstones to use in jewelry but only one makes for the perfect engagement ring.
Are granite and diamond the only network solids.
You might ask is baby power a solid.
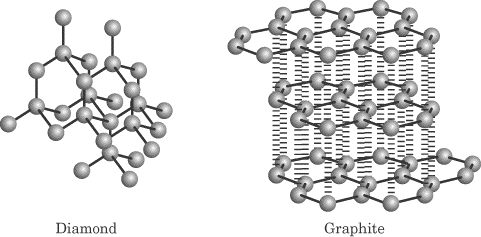
The structure of diamond is shown at the right in a ball and stick format.
Which statement best describes why graphite is soft.
Get started your new countertop in 4 easy steps.
In the diagram some carbon atoms only seem to be forming two bonds or even one bond but that s not really the case.
Carbon has an electronic arrangement of 2 4.
In diamond each carbon shares electrons with four other carbon atoms forming four single bonds.
Most covalent molecular structures have low melting and boiling points.
A graphite is made up of only carbon atoms.
The key is that solids hold their shape and they don t flow like a liquid.
It is a strong rigid three dimensional structure that results in an infinite network of atoms.
Covalent molecular compounds usually have a low enthalpy of fusion and vaporization due to the same reason.
A diamond has a crystal structure similar to ice b diamond is made up of only carbon atoms c the c c bonds all have different bond lengths d a rigid network of atoms that cannot move 4.
The enthalpy of fusion is the amount of energy that is required to melt a solid.
Visit our showroom to select your material from our wide variety of premium choices and make selections for your edge profile sink options.
This accounts for diamond s hardness extraordinary strength and durability and gives diamond a.
Take a look at our quartz vs diamond comparison as we ll discuss the similarities and differences in brilliance hardness cut and other characteristics you should look into when buying a gemstone.
Graphite ˈ ɡ r æ f aɪ t archaically referred to as plumbago is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure it occurs naturally in this form and is the most stable form of carbon under standard conditions under high pressures and temperatures it converts to diamond graphite is used in pencils and lubricants.
The same goes for a diamond.
Graphite the most stable form diamond and fullerene.
It s soft and powdery baby power is also a solid.
This is because the intermolecular forces between covalent molecules require a lower amount of energy to separate from each other.
We re dedicated to providing superior customer service and only the premier materials for stunning results every time.
One common examples of network solids are diamond a form of pure carbon carbon exists as a pure element at room temperature in three different forms.
Solids can hold their shape because their molecules are tightly packed together.
A rock will always look like a rock unless something happens to it.